Publications
 Frydendall, E. and Scott, E.E.
(2024)
Development of a high throughput cytochrome P450-ligand binding assay
J. Biol. Chem.
19:107799.
Frydendall, E. and Scott, E.E.
(2024)
Development of a high throughput cytochrome P450-ligand binding assay
J. Biol. Chem.
19:107799.
 Loomis, C.L., Im, S-C., and Scott, E.E.
(2024)
Adrenodoxin allosterically alters human cytochrome P450 11B enzymes to accelerate substrate binding and decelerate release
RSC Chem. Biol.
5:938.
Loomis, C.L., Im, S-C., and Scott, E.E.
(2024)
Adrenodoxin allosterically alters human cytochrome P450 11B enzymes to accelerate substrate binding and decelerate release
RSC Chem. Biol.
5:938.
 Richard, A.M., Estrada, D.F., Flynn, L., Pochapsky, S.S., Scott, E.E., and Pochapsky, T.C.
(2024)
Tracking protein-protein interactions by NMR: Conformational selection in human steroidogenic cytochrome P450 CYP17A1 induced by cytochrome b5
RSC Phys. Chem. Chem. Phys.
26:16980.
Richard, A.M., Estrada, D.F., Flynn, L., Pochapsky, S.S., Scott, E.E., and Pochapsky, T.C.
(2024)
Tracking protein-protein interactions by NMR: Conformational selection in human steroidogenic cytochrome P450 CYP17A1 induced by cytochrome b5
RSC Phys. Chem. Chem. Phys.
26:16980.
 Richard, A.M., Wong, N.R., Harris, K., Sundar, R., Scott, E.E., and Pochapsky, T.C.
(2023)
An approach to selective steroidogenic cytochrome P450 haem iron ligation by steroid-derived isonitriles
Commun. Chem.
6:183.
Richard, A.M., Wong, N.R., Harris, K., Sundar, R., Scott, E.E., and Pochapsky, T.C.
(2023)
An approach to selective steroidogenic cytochrome P450 haem iron ligation by steroid-derived isonitriles
Commun. Chem.
6:183.
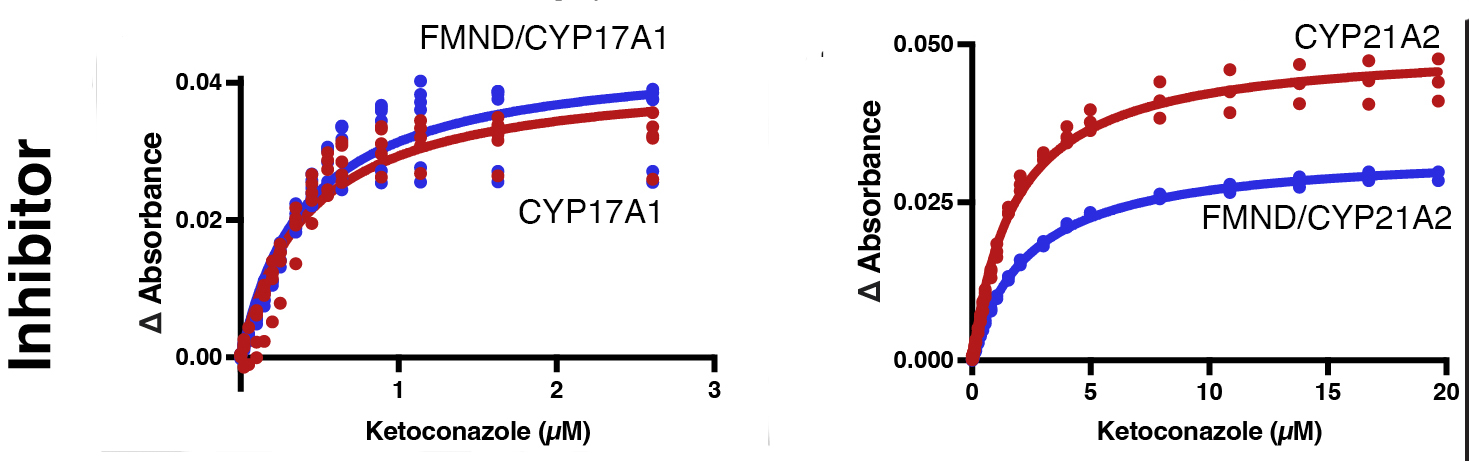 Burris-Hiday, Scott, E.E.
(2023)
Allosteric Modulation of Cytochrome P450 Enzymes by the NADPH Cytochrome P450 Reductase FMN-containing domain
J. Biol. Chem.
299:105112.
Burris-Hiday, Scott, E.E.
(2023)
Allosteric Modulation of Cytochrome P450 Enzymes by the NADPH Cytochrome P450 Reductase FMN-containing domain
J. Biol. Chem.
299:105112.
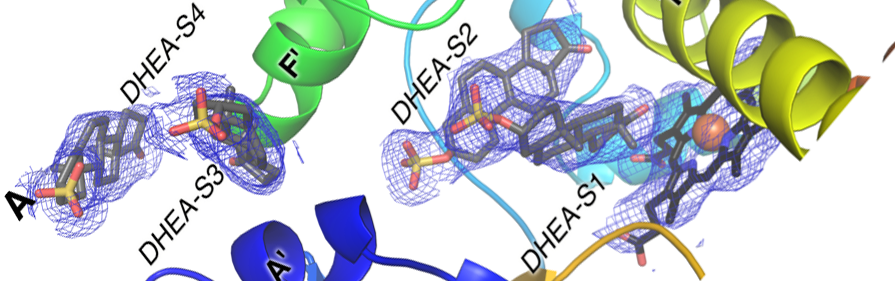 Liu,J., Kandel, S.E., Lampe, J.N., and Scott, E.E.
(2023)
Human cytochrome P450 3A7 binding four copies of Its native substrate dehydroepiandrosterone 3-sulfate
J. Biol. Chem.
299:104993.
Liu,J., Kandel, S.E., Lampe, J.N., and Scott, E.E.
(2023)
Human cytochrome P450 3A7 binding four copies of Its native substrate dehydroepiandrosterone 3-sulfate
J. Biol. Chem.
299:104993.
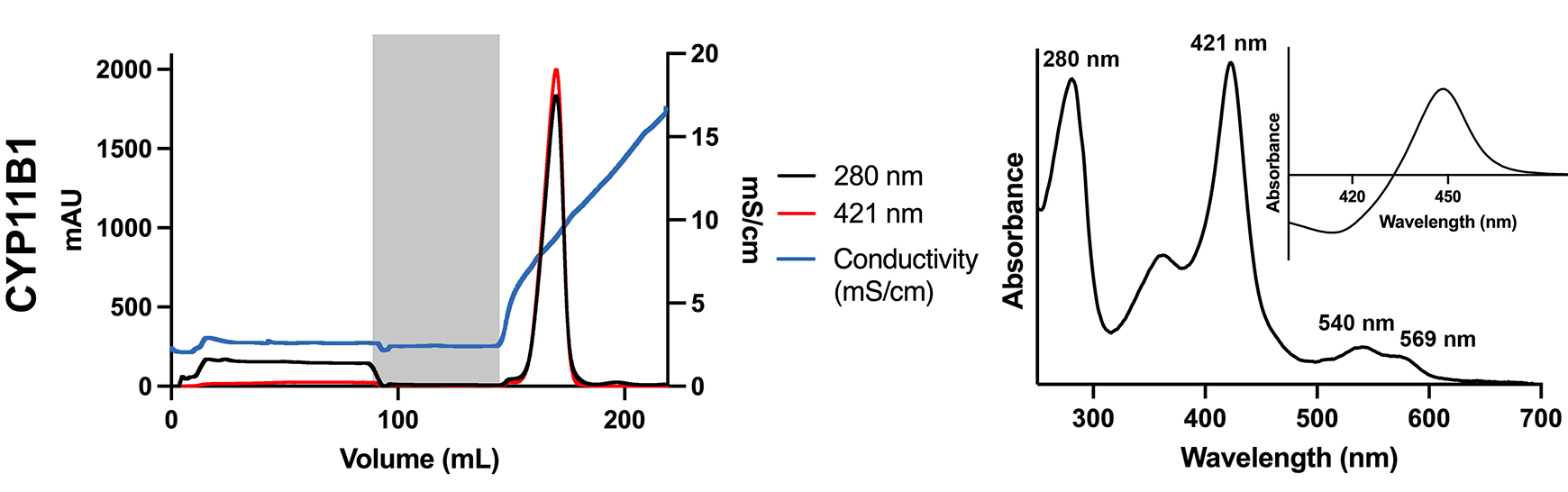 Burris-Hiday, S.D., Loomis, C.L., Richard, A.M., Scott, E.E.
(2023)
Generation of human steroidogenic cytochrome P450 enzymes for structural and functional characterization
Meth. Enzymol.
689:3.
Burris-Hiday, S.D., Loomis, C.L., Richard, A.M., Scott, E.E.
(2023)
Generation of human steroidogenic cytochrome P450 enzymes for structural and functional characterization
Meth. Enzymol.
689:3.
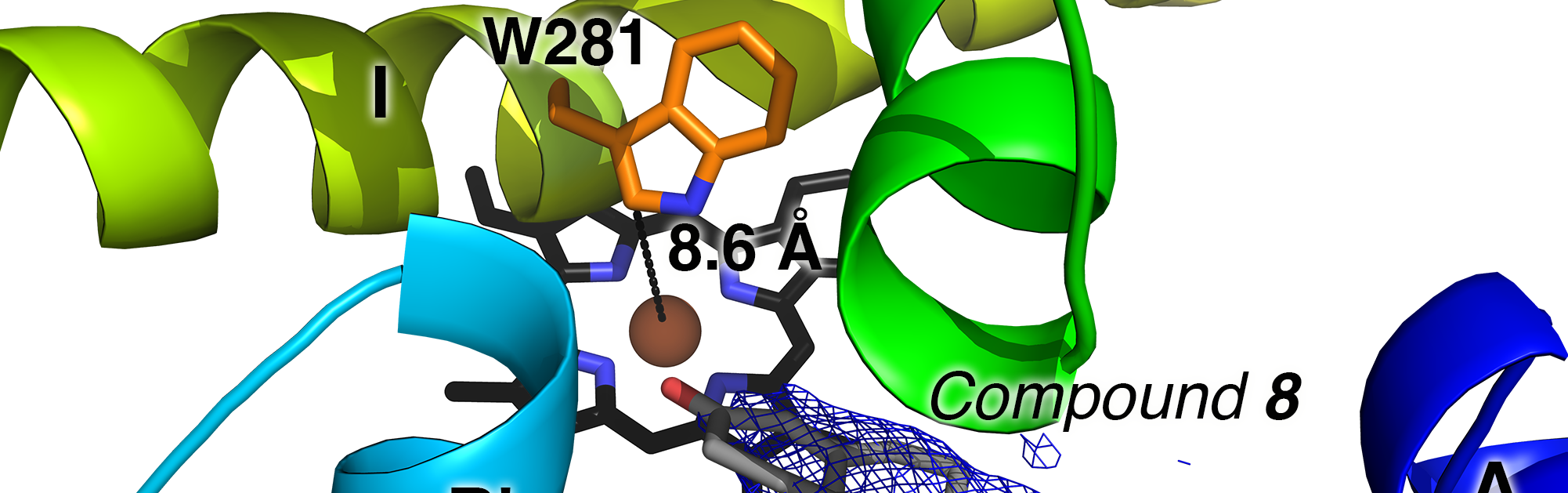 Liu, J., Offei, S.D., Yoshimoto, F.K., Scott, E.E.
(2023)
Pyridine-containing substrate analogs are restricted from accessing the human cytochrome P450 8B1 active site by tryptophan 281
J. Biol. Chem.
299:103032.
Liu, J., Offei, S.D., Yoshimoto, F.K., Scott, E.E.
(2023)
Pyridine-containing substrate analogs are restricted from accessing the human cytochrome P450 8B1 active site by tryptophan 281
J. Biol. Chem.
299:103032.
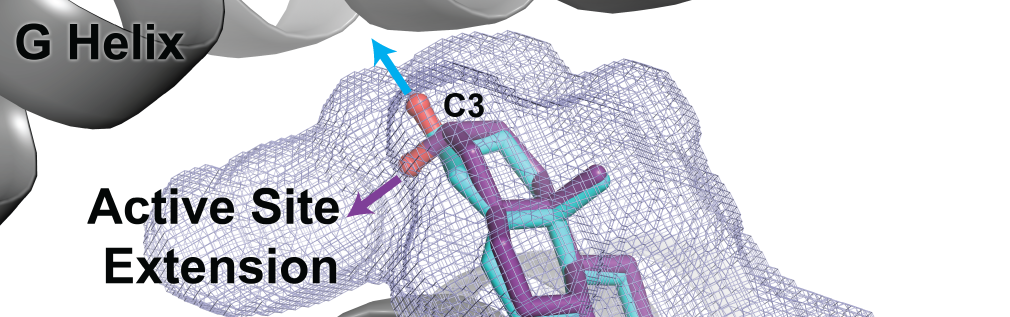 Petrunak, E.M., Bart, A.G., Peng, H-W., Auchus, R.J., and Scott, E.E.
(2023)
Human Cytochrome P450 17A1 Structures with Metabolites of Prostate Cancer Drug Abiraterone Reveal Substrate-Binding Plasticity and a Second Binding Site
J. Biol. Chem.
299(3):102999.
Petrunak, E.M., Bart, A.G., Peng, H-W., Auchus, R.J., and Scott, E.E.
(2023)
Human Cytochrome P450 17A1 Structures with Metabolites of Prostate Cancer Drug Abiraterone Reveal Substrate-Binding Plasticity and a Second Binding Site
J. Biol. Chem.
299(3):102999.
 Roberts, A.G., Stevens, J.C., Szklarz, G.D., Scott, E.E., Kumar, S., Shah, M.B., and Halpert, J.R.
(2023)
Four decades of CYP2B research: from protein adducts to protein structures and beyond
Drug Metab. Dispos.
51:111-122.
Roberts, A.G., Stevens, J.C., Szklarz, G.D., Scott, E.E., Kumar, S., Shah, M.B., and Halpert, J.R.
(2023)
Four decades of CYP2B research: from protein adducts to protein structures and beyond
Drug Metab. Dispos.
51:111-122.
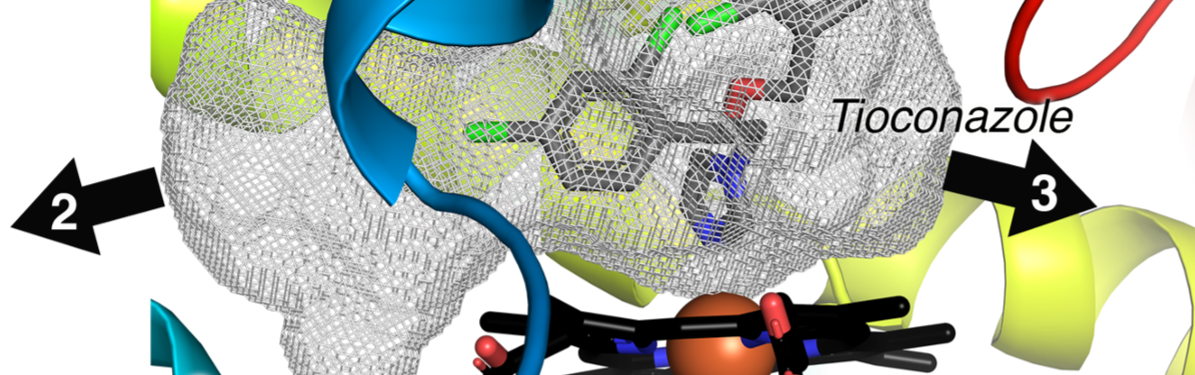 Liu, J., Carlson, H.A., and Scott, E.E.
(2022)
Cytochrome P450 8B1 structure and function: supporting drug design for non-alcoholic fatty liver disease and diabetes
J. Biol. Chem.
298:102344. *Selected as Editor's Pick*.
Liu, J., Carlson, H.A., and Scott, E.E.
(2022)
Cytochrome P450 8B1 structure and function: supporting drug design for non-alcoholic fatty liver disease and diabetes
J. Biol. Chem.
298:102344. *Selected as Editor's Pick*.
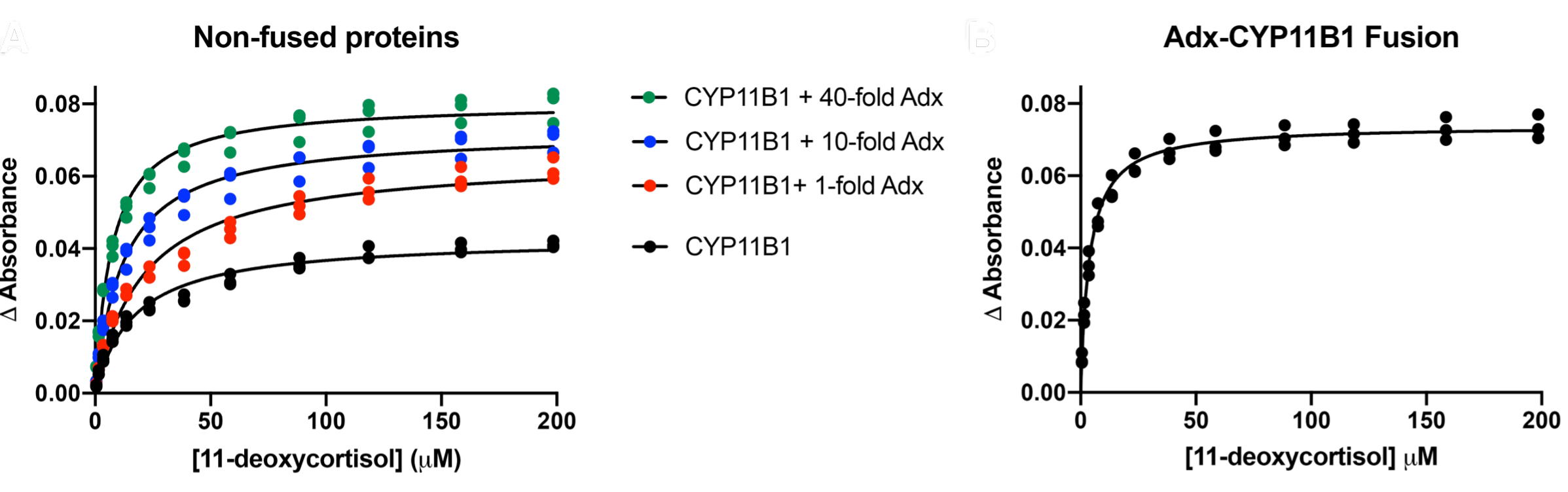 Loomis, C.L., Brixius-Anderko, S., and Scott, E.E.
(2022)
Redox partner adrenodoxin alters cytochrome P450 11B1 ligand binding and inhibition
J. Inorg. Biochem.
235:111934.
Loomis, C.L., Brixius-Anderko, S., and Scott, E.E.
(2022)
Redox partner adrenodoxin alters cytochrome P450 11B1 ligand binding and inhibition
J. Inorg. Biochem.
235:111934.
 Bart, A.G., Morais, G., Vangala, V.R., Loadman, P.M., Pors, K. and Scott, E.E.
(2022)
Cytochrome P450 binding and bioactivation of tumor-targeted duocarmycin agents
Drug Metab. Dispos.
50:49-57.
Bart, A.G., Morais, G., Vangala, V.R., Loadman, P.M., Pors, K. and Scott, E.E.
(2022)
Cytochrome P450 binding and bioactivation of tumor-targeted duocarmycin agents
Drug Metab. Dispos.
50:49-57.
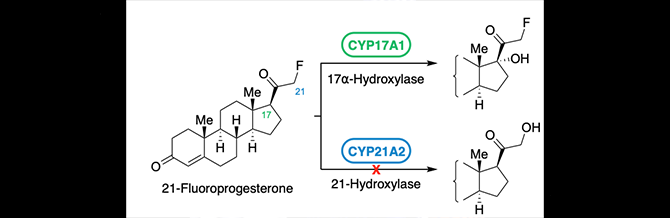 Vogt, C.D., Bart, A.G., Yadav, R., Scott, E.E. and Aubé, J.
(2021)
Effects of fluorine substitution on substrate conversion by cytochromes P450 17A1 and 21A2
Org. Biomol. Chem
19:7664-7669.
Vogt, C.D., Bart, A.G., Yadav, R., Scott, E.E. and Aubé, J.
(2021)
Effects of fluorine substitution on substrate conversion by cytochromes P450 17A1 and 21A2
Org. Biomol. Chem
19:7664-7669.
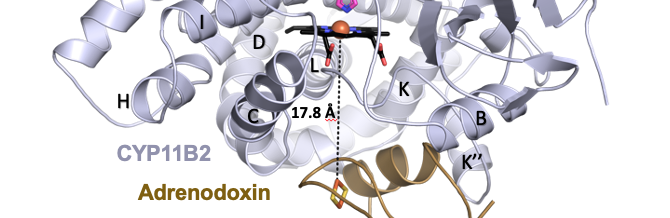 Brixius-Anderko, S. and Scott, E. E.
(2021)
Structural and functional insights into aldosterone synthase interaction with its redox partner protein adrenodoxin
J. Biol. Chem.
296:100794.
Brixius-Anderko, S. and Scott, E. E.
(2021)
Structural and functional insights into aldosterone synthase interaction with its redox partner protein adrenodoxin
J. Biol. Chem.
296:100794.
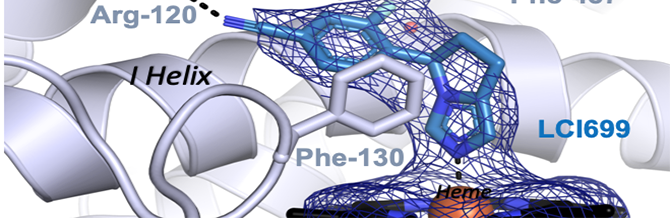 Brixius-Anderko, S. and Scott, E. E.
(2021)
Aldosterone synthase structure with Cushing’s Disease drug LCI699 highlights avenues for selective CYP11B drug design
Hypertension
78:751-759.
Brixius-Anderko, S. and Scott, E. E.
(2021)
Aldosterone synthase structure with Cushing’s Disease drug LCI699 highlights avenues for selective CYP11B drug design
Hypertension
78:751-759.
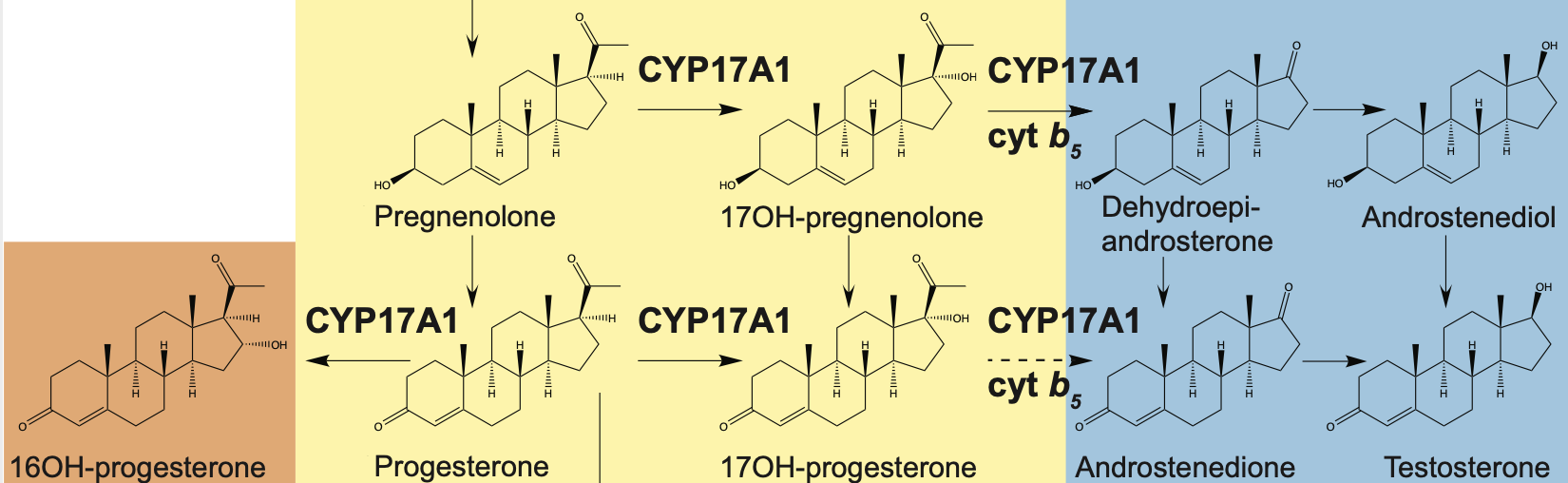 Burris-Hiday, S.B. and Scott, E.E.
(2021)
Steroidogenic cytochrome P450 17A1 structure and function
Mol. Cell. Endocrinol.
528:11261.
Burris-Hiday, S.B. and Scott, E.E.
(2021)
Steroidogenic cytochrome P450 17A1 structure and function
Mol. Cell. Endocrinol.
528:11261.
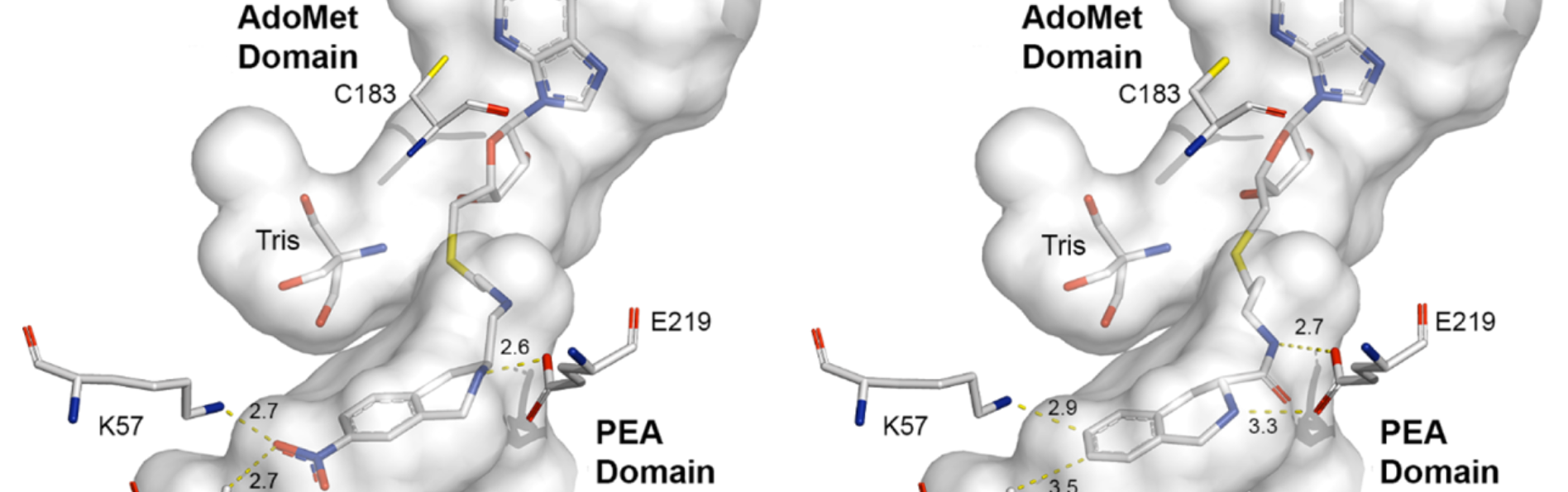 Lu, J., Bart, A.G., Wu, Q., Criscione, K.R., McLeish, M.J., Scott, E.E., and Grunewald, G.L.
(2020)
Structure-based drug design of bisubstrate inhibitors of phenylethanolamine N‐Methyltransferase possessing low nanomolar affinity at both substrate binding domains
J. Med. Chem
63:13878-13898.
Lu, J., Bart, A.G., Wu, Q., Criscione, K.R., McLeish, M.J., Scott, E.E., and Grunewald, G.L.
(2020)
Structure-based drug design of bisubstrate inhibitors of phenylethanolamine N‐Methyltransferase possessing low nanomolar affinity at both substrate binding domains
J. Med. Chem
63:13878-13898.
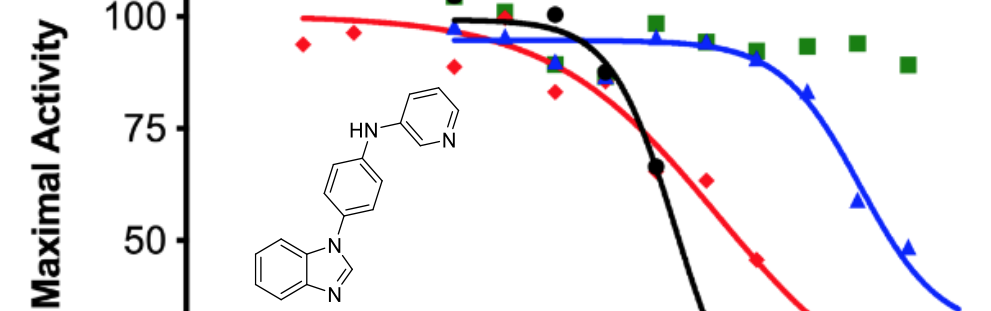 Wróbel, T. M., Rogova, O., Andersen, K. L., Yadav, R., Brixius-Anderko, S., Scott, E. E., Olsen, L., Jørgensen, F. S. and Björkling, F.
(2020)
Discovery of novel non-steroidal cytochrome P450 17A1 inhibitors as potential prostate cancer agents
Int. J. Mol. Sci.
21:4868-4879.
Wróbel, T. M., Rogova, O., Andersen, K. L., Yadav, R., Brixius-Anderko, S., Scott, E. E., Olsen, L., Jørgensen, F. S. and Björkling, F.
(2020)
Discovery of novel non-steroidal cytochrome P450 17A1 inhibitors as potential prostate cancer agents
Int. J. Mol. Sci.
21:4868-4879.
 Russell, L.E., Schleiff, M.A., Gonzalez, E., Bart, A.G., Broccatelli, F., Durmus, S., Hartman, J.H., Humphreys, W.G., Lauschke, V.M., Martin, I., Nichols, C., Nwabufo, C., Prasad, B., Scott, E.E., Segall, M., Takahashi, R., Taub, M.E., and Sodhi, J..K.
(2020)
Advances in the study of drug metabolism
Drug Metab. Rev.
295:5640-5653.
Russell, L.E., Schleiff, M.A., Gonzalez, E., Bart, A.G., Broccatelli, F., Durmus, S., Hartman, J.H., Humphreys, W.G., Lauschke, V.M., Martin, I., Nichols, C., Nwabufo, C., Prasad, B., Scott, E.E., Segall, M., Takahashi, R., Taub, M.E., and Sodhi, J..K.
(2020)
Advances in the study of drug metabolism
Drug Metab. Rev.
295:5640-5653.
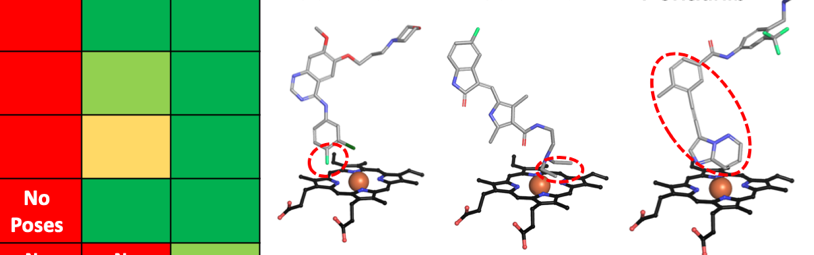
 Bart, A. G., Harris, K. L., Gillam E. M. J., and Scott, E. E.
(2020)
Structure of an ancestral mammalian family 1B1 cytochrome P450 with increased thermostability
J. Biol. Chem.
295:5640-5653.
Bart, A. G., Harris, K. L., Gillam E. M. J., and Scott, E. E.
(2020)
Structure of an ancestral mammalian family 1B1 cytochrome P450 with increased thermostability
J. Biol. Chem.
295:5640-5653.
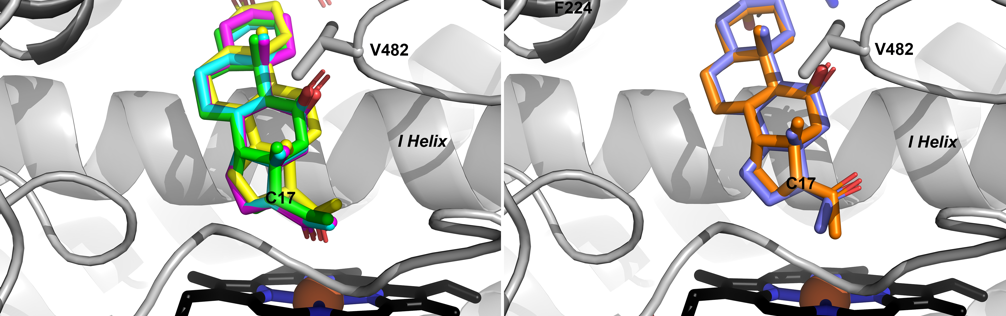 van Rooyen, D., Yadav, R., Scott, E. E. and Swart, A. C.
(2020)
CYP17A1 exhibits 17α-hydroxylase/17,20-lyase activity towards 11β-hydroxy progesterone and 11-ketoprogesterone metabolites in the C11-oxy backdoor pathway
J. Steroid Biochem. Mol. Biol.
199:105614.
van Rooyen, D., Yadav, R., Scott, E. E. and Swart, A. C.
(2020)
CYP17A1 exhibits 17α-hydroxylase/17,20-lyase activity towards 11β-hydroxy progesterone and 11-ketoprogesterone metabolites in the C11-oxy backdoor pathway
J. Steroid Biochem. Mol. Biol.
199:105614.
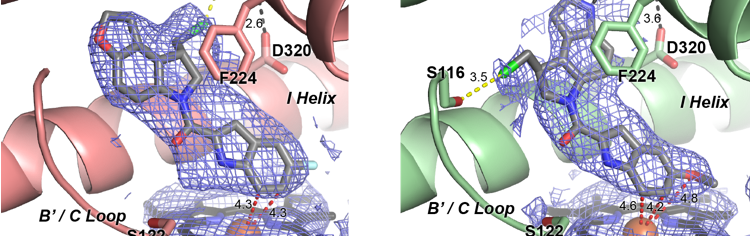 (2020)
Cytochrome P450 Binding and Activation of Anticancer Duocarmycin Produgs
J. Biol. Chem.
in press.
(2020)
Cytochrome P450 Binding and Activation of Anticancer Duocarmycin Produgs
J. Biol. Chem.
in press.
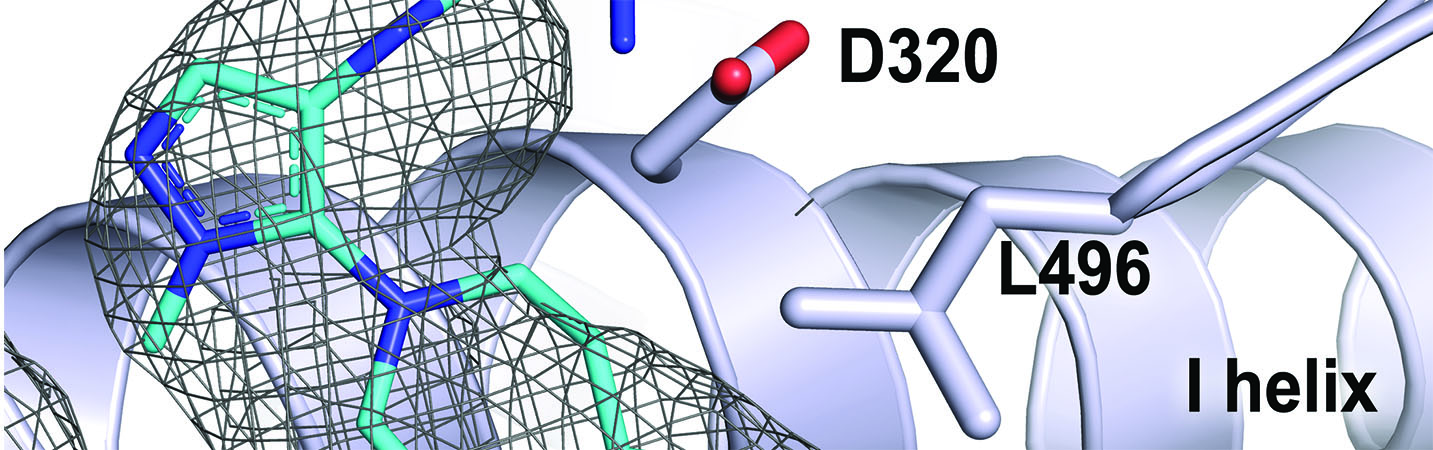 Bart A. G., Takahashi R. H., Wang X., Scott E. E.
(2019)
Human cytochrome P450 1A1 adapts active site for atypical nonplanar substrate
Drug Metab. Dispos.
48: 86-92.
Bart A. G., Takahashi R. H., Wang X., Scott E. E.
(2019)
Human cytochrome P450 1A1 adapts active site for atypical nonplanar substrate
Drug Metab. Dispos.
48: 86-92.
 Brixius-Anderko, S. and Scott, E. E.
(2019)
Structure of human cortisol-producing cytochrome P450 11B1 bound to the breast cancer drug fadrozole provides insights for drug design
J. Biol. Chem.
294: 453-460.
Brixius-Anderko, S. and Scott, E. E.
(2019)
Structure of human cortisol-producing cytochrome P450 11B1 bound to the breast cancer drug fadrozole provides insights for drug design
J. Biol. Chem.
294: 453-460.
 Bart, A. G. and Scott, E. E.
(2018)
Structures of human cytochrome P450 1A1 with bergamottin and erlotinib
J. Biol. Chem.
293:19201-19210.
Bart, A. G. and Scott, E. E.
(2018)
Structures of human cytochrome P450 1A1 with bergamottin and erlotinib
J. Biol. Chem.
293:19201-19210.
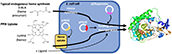 Yadav, R. and Scott, E. E.
(2018)
Endogenous insertion of non-native metalloporphyrins into human membrane cytochrome P450 enzymes
J. Biol. Chem.
293:16623-16634.
Yadav, R. and Scott, E. E.
(2018)
Endogenous insertion of non-native metalloporphyrins into human membrane cytochrome P450 enzymes
J. Biol. Chem.
293:16623-16634.
 Scott, E. E. and Godamudunage, M. P. (2018) Structures of Human Cytochrome P450 Enzymes: Variations on a Theme
(2018)
Dioxygen-dependent Heme Enzymes
Eds. Ikeda-Saito and Raven. Publisher, Royal Society of Chemistry
.
Scott, E. E. and Godamudunage, M. P. (2018) Structures of Human Cytochrome P450 Enzymes: Variations on a Theme
(2018)
Dioxygen-dependent Heme Enzymes
Eds. Ikeda-Saito and Raven. Publisher, Royal Society of Chemistry
.
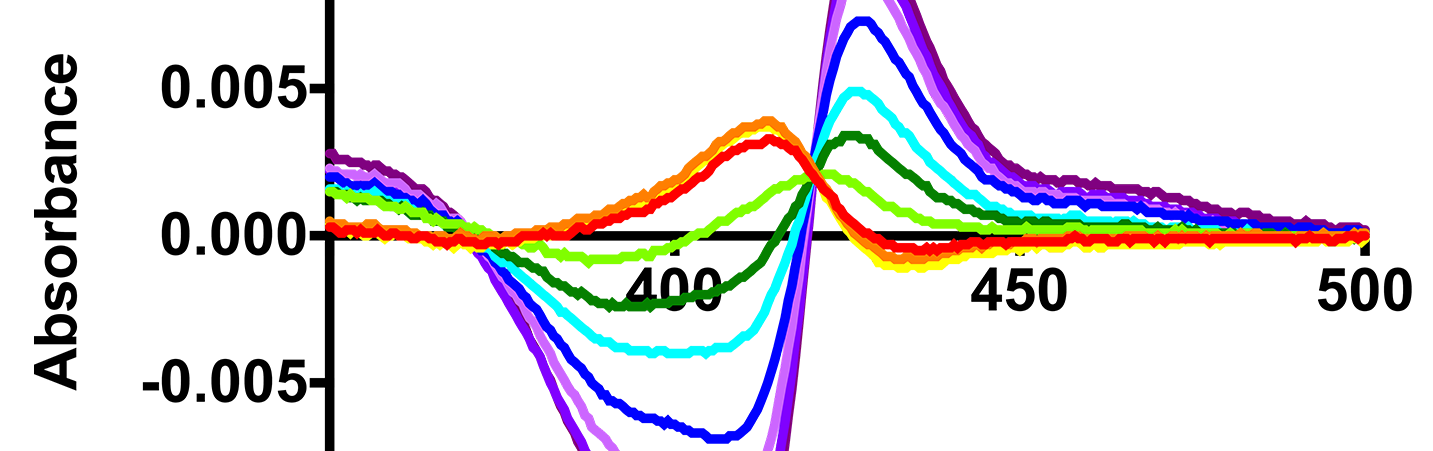 Godamudunage, M. P., Grech, A. M. and Scott, E. E.
(2018)
Comparison of antifungal azole interactions with adult cytochrome P450 3A4 vs. neonatal cytochrome P450 3A7
Drug Metab. Dispos.
46:1329-1337.
Godamudunage, M. P., Grech, A. M. and Scott, E. E.
(2018)
Comparison of antifungal azole interactions with adult cytochrome P450 3A4 vs. neonatal cytochrome P450 3A7
Drug Metab. Dispos.
46:1329-1337.
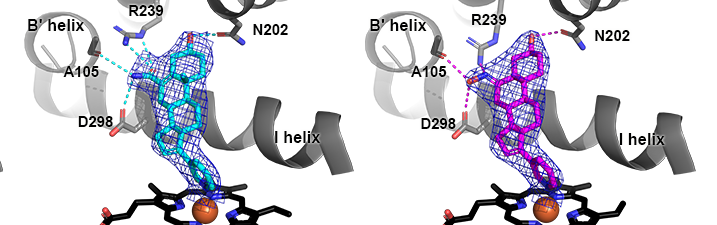 Fehl, C., Vogt, C., Yadav, R. Li, K., Scott, E. E. and Aubé, J.
(2018)
Structure-based design of inhibitors with improved selectivity for steroidogenic cytochrome P450 17A1 over cytochrome P450 21A2
J. Med. Chem
11:4946-4960.
Fehl, C., Vogt, C., Yadav, R. Li, K., Scott, E. E. and Aubé, J.
(2018)
Structure-based design of inhibitors with improved selectivity for steroidogenic cytochrome P450 17A1 over cytochrome P450 21A2
J. Med. Chem
11:4946-4960.
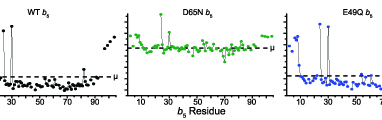 Bart, A.G. and Scott, E.E.
(2017)
Structural and functional effects of cytochrome b5 interactions with human cytochrome P450 enzymes
J. Biol. Chem.
20818-20833.
Bart, A.G. and Scott, E.E.
(2017)
Structural and functional effects of cytochrome b5 interactions with human cytochrome P450 enzymes
J. Biol. Chem.
20818-20833.
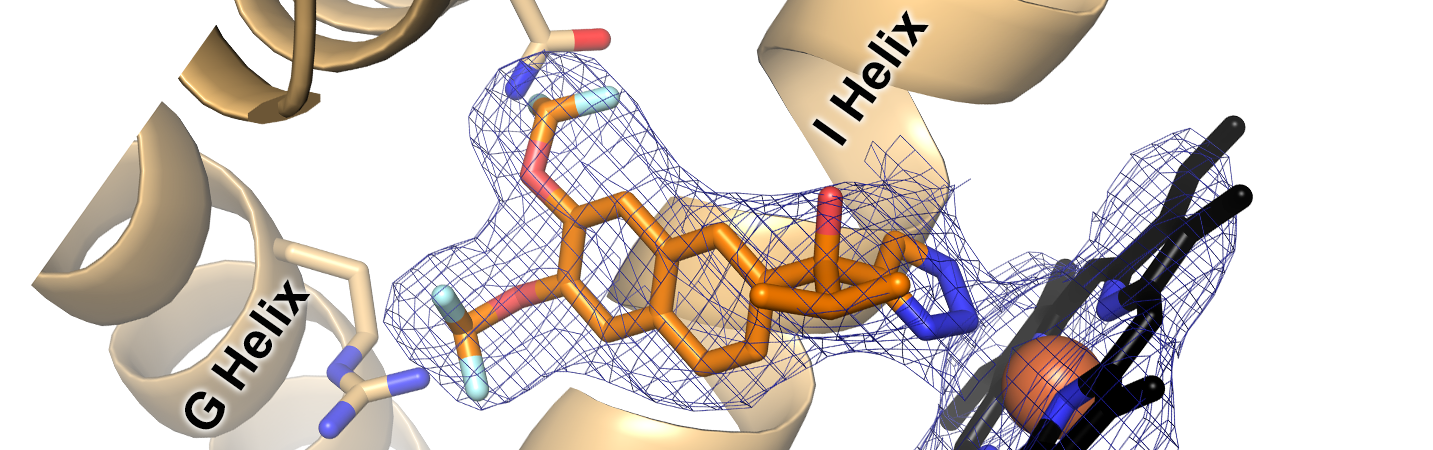 Petrunak, E.M., Rogers, S.A., Aubé, J., and Scott, E.E.
(2017)
Structural and functional evaluation of clinically relevant inhibitors of cytochrome P450 17A1 (CYP17A1)
Drug Metab. Dispos.
635-645.
Petrunak, E.M., Rogers, S.A., Aubé, J., and Scott, E.E.
(2017)
Structural and functional evaluation of clinically relevant inhibitors of cytochrome P450 17A1 (CYP17A1)
Drug Metab. Dispos.
635-645.
 Scott, E.E.
(2017)
Omega versus omega-1 hydroxylation: Cytochrome P450 4B1 sterics make the call
J. Biol. Chem.
292:5622-5623.
Scott, E.E.
(2017)
Omega versus omega-1 hydroxylation: Cytochrome P450 4B1 sterics make the call
J. Biol. Chem.
292:5622-5623.
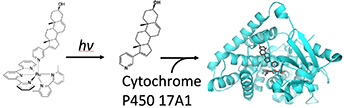 Li, A., Yadav, R., White, J.K., Herroon, M.K., Callahan, B.P., Podgorski, I., Turro, C., Scott, E.E., and Kodanko, J.J.
(2017)
Illuminating cytochrome P450 binding: Ru (II)-caged inhibitors of CYP17A1
Commun. Chem.
53:3673-3676.
Li, A., Yadav, R., White, J.K., Herroon, M.K., Callahan, B.P., Podgorski, I., Turro, C., Scott, E.E., and Kodanko, J.J.
(2017)
Illuminating cytochrome P450 binding: Ru (II)-caged inhibitors of CYP17A1
Commun. Chem.
53:3673-3676.
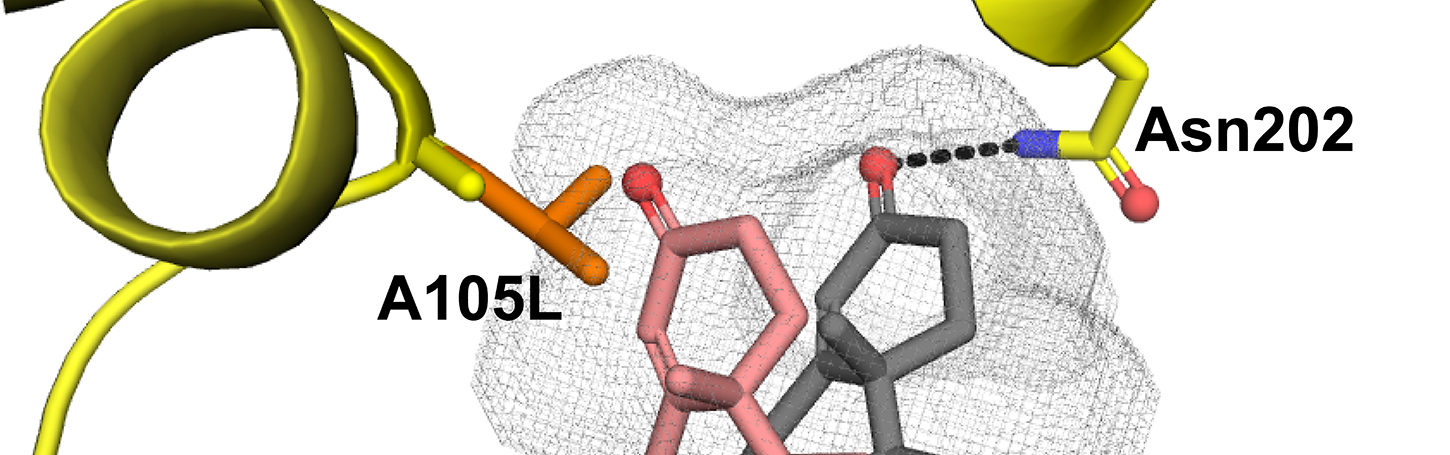 Yadav, R., Petrunak, E.M., Estrada, D.F., and Scott, E.E.
(2016)
Structural insights into the function of steroidogenic cytochrome P450 17A1
Mol. Cell. Endocrinol.
7207:30330-30336.
Yadav, R., Petrunak, E.M., Estrada, D.F., and Scott, E.E.
(2016)
Structural insights into the function of steroidogenic cytochrome P450 17A1
Mol. Cell. Endocrinol.
7207:30330-30336.
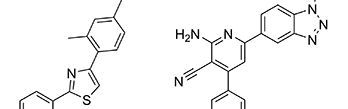 Bonomo, S., Hansen, C.H., Petrunak, E.M., Scott, E.E., Styrishave, B., Jorgensen, F. S., and Olsen, L.
(2016)
Promising tools in prostate cancer research: Selective non steroidal cytochrome P450 17A1 inhibitors
Nat. Sci. Reports
6:29468-29479.
Bonomo, S., Hansen, C.H., Petrunak, E.M., Scott, E.E., Styrishave, B., Jorgensen, F. S., and Olsen, L.
(2016)
Promising tools in prostate cancer research: Selective non steroidal cytochrome P450 17A1 inhibitors
Nat. Sci. Reports
6:29468-29479.
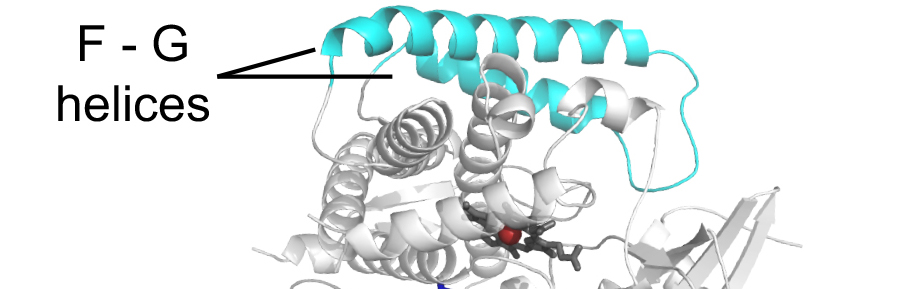 Scott, E.E., Wolf, R.C., Otyepka, M., Humphreys, S.C., Reed, J.R., Henderson, C.J., McLaughlin, L.A., Paloncýová, M., Navrátilová, V., Berka, K., Anzenbacher, P., Dahal, U.P. Barnaba, C., Brozik, J.A., Jones, J.P., Estrada, D.F., Laurence, J.S., Park, J.W., and Backes, W.L.
(2016)
The role of protein-protein and protein-membrane interactions on P450 function
Drug Metab. Dispos.
44:576-590.
Scott, E.E., Wolf, R.C., Otyepka, M., Humphreys, S.C., Reed, J.R., Henderson, C.J., McLaughlin, L.A., Paloncýová, M., Navrátilová, V., Berka, K., Anzenbacher, P., Dahal, U.P. Barnaba, C., Brozik, J.A., Jones, J.P., Estrada, D.F., Laurence, J.S., Park, J.W., and Backes, W.L.
(2016)
The role of protein-protein and protein-membrane interactions on P450 function
Drug Metab. Dispos.
44:576-590.
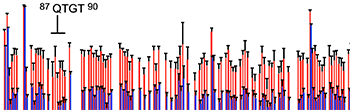 Estrada, D.F., Laurence, J.S., and Scott, E.E.
(2015)
Cytochrome P450 17A1 interactions with the FMN domain of its reductase as characterized by NMR
J. Biol. Chem.
291:3390-4003.
Estrada, D.F., Laurence, J.S., and Scott, E.E.
(2015)
Cytochrome P450 17A1 interactions with the FMN domain of its reductase as characterized by NMR
J. Biol. Chem.
291:3390-4003.
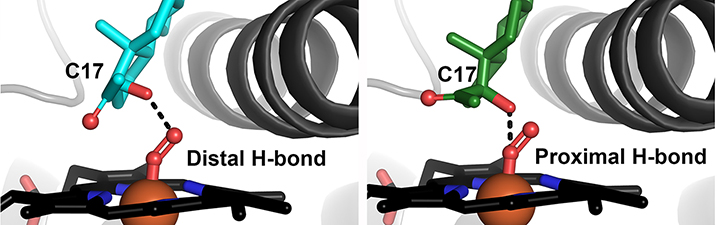 Petrunak, E.M., DeVore, N.M., Porubsky, P.R. and Scott, E.E.
(2014)
Structures of human steroidogenic cytochrome P450 17A1 with substrates
J. Biol. Chem.
289:32952-32964.
Petrunak, E.M., DeVore, N.M., Porubsky, P.R. and Scott, E.E.
(2014)
Structures of human steroidogenic cytochrome P450 17A1 with substrates
J. Biol. Chem.
289:32952-32964.
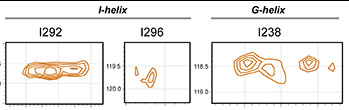 Estrada, D.F., Skinner, A.L., Laurence, J.S., and Scott, E.E.
(2014)
Human cytochrome P450 conformational selection: Modulation by ligand and cytochrome b5
J. Biol. Chem.
289:14310-14320.
Estrada, D.F., Skinner, A.L., Laurence, J.S., and Scott, E.E.
(2014)
Human cytochrome P450 conformational selection: Modulation by ligand and cytochrome b5
J. Biol. Chem.
289:14310-14320.
 Johnson, E.F., Connick, J.P., Reed, J.R., Backes, W.L., Desai, M.C., Xu, L., Estrada, D.F., Laurence, J.S. and Scott, E.E.
(2014)
Correlating structure and function of drug metabolizing enzymes: progress and ongoing challenges
Drug Metab. Dispos.
42:9-22.
Johnson, E.F., Connick, J.P., Reed, J.R., Backes, W.L., Desai, M.C., Xu, L., Estrada, D.F., Laurence, J.S. and Scott, E.E.
(2014)
Correlating structure and function of drug metabolizing enzymes: progress and ongoing challenges
Drug Metab. Dispos.
42:9-22.
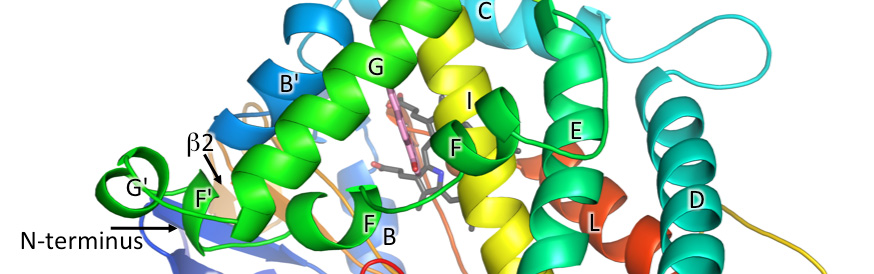 Walsh, A.A., Szklarz, G.D. and Scott, E.E.
(2013)
Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism
J. Biol. Chem.
288:12932-12943.
Walsh, A.A., Szklarz, G.D. and Scott, E.E.
(2013)
Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism
J. Biol. Chem.
288:12932-12943.
 Blake, L.C., Roy, A., Neul, D., Schoenen, F.J., Aubé, J. and Scott, E.E.
(2013)
Benzylmorpholine analogs as selective inhibitors of lung cytochrome P450 2A13 for the chemoprevention of lung cancer in tobacco users
Pharm. Res.
30:2290-2302.
Blake, L.C., Roy, A., Neul, D., Schoenen, F.J., Aubé, J. and Scott, E.E.
(2013)
Benzylmorpholine analogs as selective inhibitors of lung cytochrome P450 2A13 for the chemoprevention of lung cancer in tobacco users
Pharm. Res.
30:2290-2302.
 Estrada, D.F., Laurence, J.S., and Scott, E.E.
(2013)
Substrate-modulated cytochrome P450 17A1 and cytochrome b5 interactions revealed by NMR
J. Biol. Chem.
288:17008-17018.
Estrada, D.F., Laurence, J.S., and Scott, E.E.
(2013)
Substrate-modulated cytochrome P450 17A1 and cytochrome b5 interactions revealed by NMR
J. Biol. Chem.
288:17008-17018.
 Stephens, E.S., Walsh, A.A., and Scott, E.E.
(2012)
Evaluation of inhibition selectivity for human cytochrome P450 2A enzymes
Drug Metab. Dispos.
40:1797-802.
Stephens, E.S., Walsh, A.A., and Scott, E.E.
(2012)
Evaluation of inhibition selectivity for human cytochrome P450 2A enzymes
Drug Metab. Dispos.
40:1797-802.
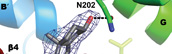 DeVore, N.M. and Scott, E.E.
(2012)
Cytochrome P450 17A1 structures with prostate cancer drugs abiraterone and TOK-001
Nature
482:116-119.
DeVore, N.M. and Scott, E.E.
(2012)
Cytochrome P450 17A1 structures with prostate cancer drugs abiraterone and TOK-001
Nature
482:116-119.
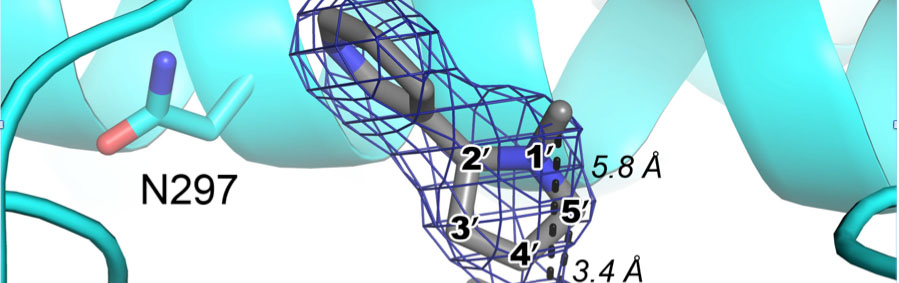 DeVore, N.M. and Scott, E.E.
(2012)
Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) binding and access channel in human cytochrome P450 2A6 and 2A13 enzymes
J. Biol. Chem.
287:26576-26585.
DeVore, N.M. and Scott, E.E.
(2012)
Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) binding and access channel in human cytochrome P450 2A6 and 2A13 enzymes
J. Biol. Chem.
287:26576-26585.
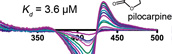 DeVore, N.M., Meneely, K.M., Bart, A.G., Stephens, E.S., Battaile, K.P., and Scott, E.E.
(2012)
Structural comparison of cytochromes P450 2A6, 2A13, and 2E1 with pilocarpine
FEBS J.
279:1621-31 .
DeVore, N.M., Meneely, K.M., Bart, A.G., Stephens, E.S., Battaile, K.P., and Scott, E.E.
(2012)
Structural comparison of cytochromes P450 2A6, 2A13, and 2E1 with pilocarpine
FEBS J.
279:1621-31 .
 Reed, T., Lushington, G.H., Xia, Y., Hirakawa, H., Mure, M., Scott, E.E., and Limburg, J.
(2010)
Crystal structure of histamine dehydrogenase from Nocardioides simplex
J. Biol. Chem.
285:25782-25791.
Reed, T., Lushington, G.H., Xia, Y., Hirakawa, H., Mure, M., Scott, E.E., and Limburg, J.
(2010)
Crystal structure of histamine dehydrogenase from Nocardioides simplex
J. Biol. Chem.
285:25782-25791.
 Porubsky, P.R., Battaile, K.P., and Scott, E.E.
(2010)
Human cytochrome P450 2E1 structures with fatty acid analogs reveal a previously unobserved binding mode
J. Biol. Chem.
285:22282-22290.
Porubsky, P.R., Battaile, K.P., and Scott, E.E.
(2010)
Human cytochrome P450 2E1 structures with fatty acid analogs reveal a previously unobserved binding mode
J. Biol. Chem.
285:22282-22290.
 Swanson, H.I., Njar, V.C.O., Yu, Z., Castro, D.J., Gonzalez, F.J., Williams, D.E., Huang, Y., Kong, A-N.T., Doloff, J.C., Ma, J., Waxman, D.J., and Scott, E.E.
(2010)
Targeting drug metabolizing enzymes for effective chemoprevention and chemotherapy
Drug Metab. Dispos.
38:539-544.
Swanson, H.I., Njar, V.C.O., Yu, Z., Castro, D.J., Gonzalez, F.J., Williams, D.E., Huang, Y., Kong, A-N.T., Doloff, J.C., Ma, J., Waxman, D.J., and Scott, E.E.
(2010)
Targeting drug metabolizing enzymes for effective chemoprevention and chemotherapy
Drug Metab. Dispos.
38:539-544.
 Culpepper, M.A., Scott, E.E., and Limburg, J.
(2010)
Crystal structure of prolyl 4-hydroxylase from Bacillus anthracis
Biochemistry
49:124-133.
Culpepper, M.A., Scott, E.E., and Limburg, J.
(2010)
Crystal structure of prolyl 4-hydroxylase from Bacillus anthracis
Biochemistry
49:124-133.
 DeVore, N.M., Smith, B.D., Wang, J.L., Lushington, G.H., and Scott, E.E.
(2009)
Key residues controlling binding of diverse ligands to human cytochrome P450 2A Enzymes
Drug Metab. Dispos.
37:1319-1327.
DeVore, N.M., Smith, B.D., Wang, J.L., Lushington, G.H., and Scott, E.E.
(2009)
Key residues controlling binding of diverse ligands to human cytochrome P450 2A Enzymes
Drug Metab. Dispos.
37:1319-1327.
 Porubsky, P.R., Meneely, K.M., and Scott, E.E.
(2008)
Structures of human cytochrome P450 2E1: Insights into the binding of inhibitors and both small molecular weight and fatty acid substrates
J. Biol. Chem.
283:33698-33707.
Porubsky, P.R., Meneely, K.M., and Scott, E.E.
(2008)
Structures of human cytochrome P450 2E1: Insights into the binding of inhibitors and both small molecular weight and fatty acid substrates
J. Biol. Chem.
283:33698-33707.
 DeVore, N.M., Smith, B.D., Urban, M.J., and Scott, E.E.
(2008)
Key residues controlling phenacetin metabolism by human cytochrome P450 2A enzymes
Drug Metab. Dispos.
36:2582-2590.
DeVore, N.M., Smith, B.D., Urban, M.J., and Scott, E.E.
(2008)
Key residues controlling phenacetin metabolism by human cytochrome P450 2A enzymes
Drug Metab. Dispos.
36:2582-2590.
 Miller, M.A., Scott, E.E., and Limberg, J.
(2008)
Expression, purification, crystallization and preliminary X-ray studies of a proly-4-hydroxylase protein from Bacillus anthracis
Acta Crystallogr. F.
64:788-791.
Miller, M.A., Scott, E.E., and Limberg, J.
(2008)
Expression, purification, crystallization and preliminary X-ray studies of a proly-4-hydroxylase protein from Bacillus anthracis
Acta Crystallogr. F.
64:788-791.
 Reed, T.M., Hirakawa, H., Mure, M., Scott, E.E., and Limberg, J.
(2008)
Expression, purification and crystallization and preliminary X-ray studies of histamine dehydrogenase from Nocardiodes simplex
Acta Crystallogr. F.
64:785-787.
Reed, T.M., Hirakawa, H., Mure, M., Scott, E.E., and Limberg, J.
(2008)
Expression, purification and crystallization and preliminary X-ray studies of histamine dehydrogenase from Nocardiodes simplex
Acta Crystallogr. F.
64:785-787.
 Porubsky, P.R., Scott, E.E., and Williams, T.D.
(2008)
p-Dimethylaminocinnamaldehyde derivatization for colorimetric detection and HPLC-UV/Vis-MS/MS identification of indoles
Arch. Biochem. Biophys.
475:14-17.
Porubsky, P.R., Scott, E.E., and Williams, T.D.
(2008)
p-Dimethylaminocinnamaldehyde derivatization for colorimetric detection and HPLC-UV/Vis-MS/MS identification of indoles
Arch. Biochem. Biophys.
475:14-17.
 Schlicht, K.E., Michno, N., Smith, B.D., Scott, E.E., and Murphy, S.E.
(2007)
Functional characterization of CYP2A13 polymorphisms
Xenobiotica
37:1439-1349.
Schlicht, K.E., Michno, N., Smith, B.D., Scott, E.E., and Murphy, S.E.
(2007)
Functional characterization of CYP2A13 polymorphisms
Xenobiotica
37:1439-1349.
 Smith B.D., Sanders J.L., Porubsky P.R., Lushington G.H., Stout C.D., and Scott, E.E.
(2007)
Structure of the human lung cytochrome P450 2A13
J. Biol. Chem.
282:17306-17313.
Smith B.D., Sanders J.L., Porubsky P.R., Lushington G.H., Stout C.D., and Scott, E.E.
(2007)
Structure of the human lung cytochrome P450 2A13
J. Biol. Chem.
282:17306-17313.
 Scott E.E. and Halpert J.R.
(2005)
Structures of cytochrome P450 3A4
Trends in Biochem. Sci.
30:5-7.
Scott E.E. and Halpert J.R.
(2005)
Structures of cytochrome P450 3A4
Trends in Biochem. Sci.
30:5-7.